Types of Thermodynamic System with Example [PDF]
Hello readers, welcome Mechanical Students, today in this paper we will be going to discuss on the Types of Thermodynamic System with Proper Example. So let's see what is a Thermodynamic System?
Thermodynamic System:
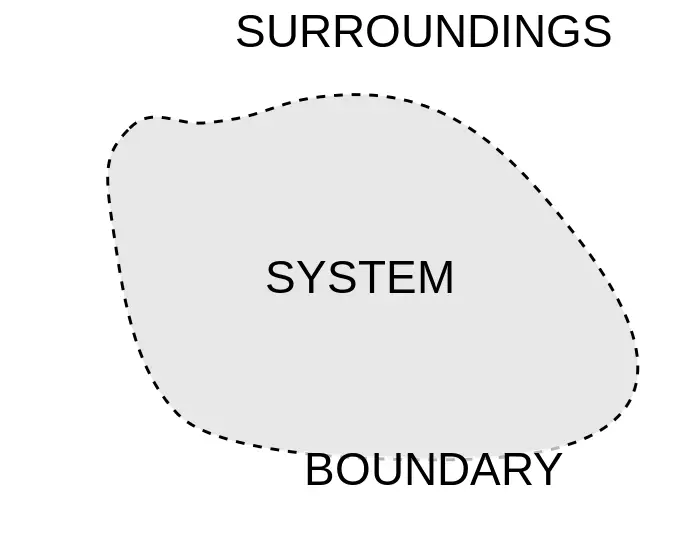
An enclosed space that has been separated for analysis, observation, and inference is called a system.
or
A Thermodynamic system is defined as a finite quantity of matter or prescribed region of space.
Now let's see,
What is Boundary, Surroundings, and Universe of a Thermodynamic system?
What is boundary of a system?
It is a real or imaginary envelope enclosing a system called boundary of a system.
What is Surrounding in thermodynamics?
Everything external to the system is called Surroundings of a Thermodynamic system.
What is universe in thermodynamics?
System and its surroundings together comprise a Universe.
Before entering into the concept of the types of a thermodynamic system, we need to know a few concepts which are related to them and are as follows.
Properties of the system:
The characteristics by which the physical condition of the system is described called properties of the system.
There are two properties of a system.
Extensive Property:
Any property that is dependent on mass is called as Extensive property.
For example, Volume, Enthalpy, Internal energy, Entropy, Kinetic energy, Potential energy etc.
Intensive Property:
Any property that is independent of mass is called an Intensive property.
For example, Pressure, Temperature, and Density, etc.
Now, Let's discuss three types of Thermodynamic System with examples.
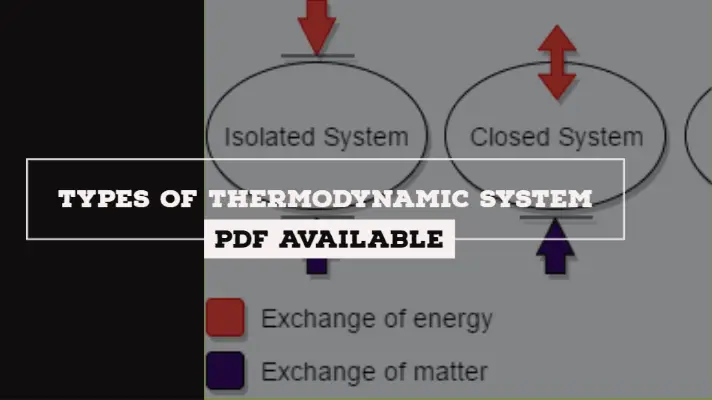
Types of Thermodynamic System:
There are three types of Thermodynamic System:
- Open System
- Closed system
- Isolated System
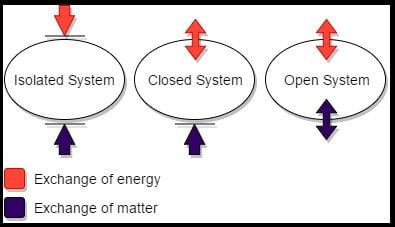
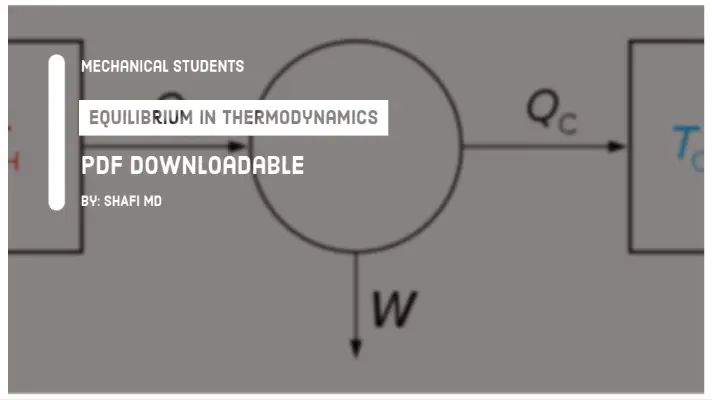
1. Open System:
It is a system in which both mass interaction as well as energy interaction takes place.
For example, Water flowing in a pipeline line [Mass and K.E of water].
2. Closed System:
It is a system in which there is only energy interaction takes place but not mass interaction.
For example, Sun
3. Isolated Systems:
It is a system in which neither mass interaction nor energy interaction takes place.
For example, Coffee in a thermos flask.
These are some common phenomena related to Thermodynamic Systems:
- A fountain pen while writing: Open system.
- Candle flame while burning: Open system.
- A TV switched ON: Closed System.
- A transformer while working: Closed System.
- Biogas Digestor: Open System.
- The moon revolves around the earth: Closed System.
- Air Conditioned Railway Coach: Open System.
Some FAQs on This Topic:
What are the different types of thermodynamic system?
What is thermodynamic system and its types?
So I hope you liked this paper on Types of Thermodynamic System, if so then don't forget to share this stuff with your friends.
References [External Links]:
- Thermodynamic System - an overview
- Thermodynamic Systems - Physics LibreTexts