Equilibrium in Thermodynamics [Definition, Types with Example] (PDF)
Hello readers, in this article we will be going to discuss the Equilibrium in Thermodynamics, and then also see the types of Equilibrium in Thermodynamics with Proper Examples, so I hope your concept will be clear after reading this paper.
Definition of Thermodynamic Equilibrium:
A system is said to be in equilibrium if it does not tend to undergo any change. Systems under Pressure and Temperature equilibrium but not under chemical equilibrium are said to be in meta-stable equilibrium condition.
Types of Thermodynamic Equilibrium with Example:
There are Four types of Thermodynamic Equilibrium, those are:
- Mechanical equilibrium
- Chemical equilibrium
- Thermal equilibrium and
- Thermodynamic equilibrium
Let me explain those one by one.
Mechanical Equilibrium:
In a system, if there are no external and internal forces acting on the system then the system is said to be in Mechanical Equilibrium.
Mechanical Equilibrium Example:
The person pressing a spring to a particular point is an example of Mechanical Equilibrium.
Chemical Equilibrium:
For a system to be in chemical equilibrium there should be equality of chemical potential, i.e., there should not be any chemical reactions.
Example of Chemical Equilibrium:
The example is cooldrink in a plastic bottle.
As you know that there is carbon dioxide that was dissolved in the liquid and also present in between the space of liquid and cap of the bottle.
There is a movement of carbon dioxide from the liquid phase to the gas phase and vice versa but if you look at the bottle, there does not appear to be any change in the bottle. It indicates that the system is said to be in Chemical Equilibrium.
Thermal Equilibrium:
For a system to be in thermal equilibrium, there should not be any temperature change in the system. (Or) The temperature across every section is the same, then the system is in Thermal Equilibrium.
Example of Thermal Equilibrium:
A Volcano and an Atmosphere are the perfect examples of Thermodynamic equilibrium i.e. when the rock or stones comes out from the molten volcano, the rock will give off its heat to the surroundings until they (rock and air) reaches the same temperature.
Thermodynamic Equilibrium:
When a system satisfies the conditions of mechanical equilibrium, chemical equilibrium, and thermal equilibrium, it is said to be in a state of thermodynamic equilibrium.
Example of Thermal Equilibrium:
A gas in a cylinder piston(movable) mechanism was said to be in equilibrium, If the pressure and temperature are uniform.
This is the explanation of Equilibrium in Thermodynamics. Lets understand Quasistatic Process.
Quasistatic Process:
A system is made to undergo a series of changes of states such that it is in thermodynamic equilibrium at each and every state. Then the locus of all these states is called a quasistatic process in detail.
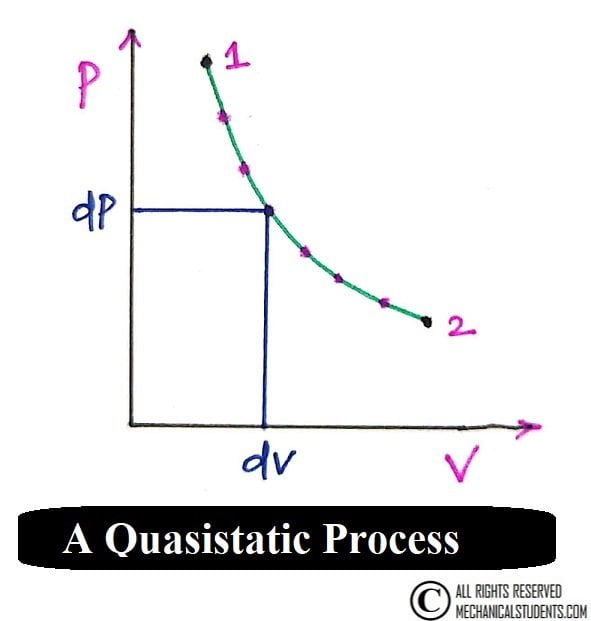
Example of Quasistatic Process:
If sand grains are allowed to fall one after the other on a piston-cylinder mechanism, then the movement of such a piston is called a quasistatic process.
It is an ideal and very slow process.
Assumptions of Ideal gas:
- Molecular separation is very high.
- Molecules do not occupy any volume.
- The molecular collisions are elastic in nature.
- There are no intermolecular forces of attraction are present.
The equation of Ideal gas is given as:
PV=mR*T
Where
P -Pressure(pascals)
V -Volume(m^3)
m -mass(Kg)
R -Gas Constant(J/kgK)
T -Temp(K)
So I hope you liked this article, if you have any doubts regarding this concept of Equilibrium in Thermodynamics, feel free to ask us in the comment section, and don't forget to share this stuff with your friends.
FAQ:
What is the difference between thermal equilibrium and thermodynamic equilibrium?
What happens when thermal equilibrium is reached?
What is thermal equilibrium example?
More Resources:
Types of Thermodynamic System
Macroscopic and Microscopic Properties [approach] of a Thermodynamic System
References [External Links]:
- A Thermodynamic Consideration of Mechanical Equilibrium
- Thermodynamic equilibrium | physics | Britannica