What is the Difference Between Diathermic and Adiabatic Process? [PDF]
Download the PDF below!
The Diathermic and Adiabatic processes are generally used in thermodynamics for explaining the behavior of the system with the surroundings. Therefore, In this article, I am going to explain the difference between the Diathermic and Adiabatic Process in a detailed manner.
But before that, lets know the Definition of Temperature...
Definition of Temperature:
It is an intensive thermodynamic property related to the “hotness” or “coldness” of a body measured on a definite scale.
Example: Thermometer.

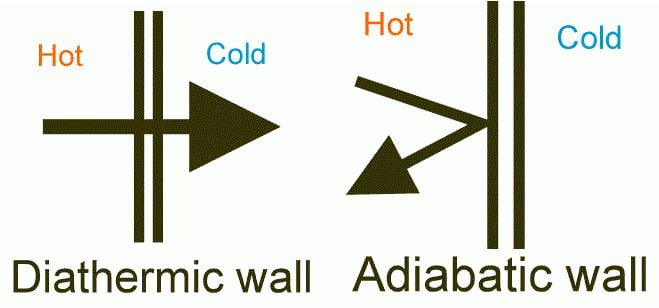
Difference Between Diathermic and Adiabatic Process:
The main differences between Diathermic and Adiabatic Process are mentioned in the tabular column below.
| Diathermic Process | Adiabatic Process |
|---|---|
| Diathermic substances are those substances that allow heat to pass through them and the process is called as Diathermic Process. | Adiabatic substances are those substances that do not allow heat to pass through them and the process is called as Adiabatic Process. |
| Any good conductor of heat is an example of diathermic substances. For example, Copper, Silver, Steel, etc. | Any Insulator, glass, wool, asbestos, cork, etc. |
This is the explanation on Diathermic and adiabatic process. If you have any doubts, you can ask us and we will give you the reply soon.
More Resources:
Macroscopic and Microscopic Properties [approach] of a Thermodynamic System [With PDF]
References [External Links]:
- Thermodynamics-Ncert